CMAJ • March 1, 2005; 172 (5_suppl).
doi:10.1503/cmaj.1040302.
© 2005 CMA Media Inc. or its
licensors
Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis
Albert E.
Chudley, Julianne Conry, Jocelynn L. Cook,
Christine Loock, Ted Rosales and Nicole
LeBlanc
From the Children's Hospital,
Health Sciences Centre, Departments of Pediatrics and Child Health and
Biochemistry and Medical Genetics, University of Manitoba, Winnipeg, Man.
(Chudley); the Department of Educational and Counselling Psychology and Special
Education, University of British Columbia; Asante Centre for Fetal Alcohol
Syndrome, Maple Ridge, B.C. (Conry); the Department of Obstetrics and
Gynecology, University of Ottawa, Ottawa, Ont. (Cook); the Department of
Pediatrics, University of British Columbia, BC Children's Hospital, Vancouver,
B.C. (Loock); the Provincial Medical Genetics Program; the Department of
Pediatrics, Memorial University of Newfoundland, St. John's, Nfld. (Rosales);
and the Department of Pediatrics, Georges Dumont Hospital, Moncton, N.B.
(LeBlanc).
Correspondence to:
Dr. Jocelynn L. Cook, FASD Team, Public Health Agency of Canada, Division of
Childhood and Adolescence, Jeanne Mance Bldg. 9th flr, Tunney's Pasture, Address
Locator 1909C2, Ottawa ON K1A 0K9; jocelynn@primus.ca
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Abstract
|
THE
DIAGNOSIS OF FETAL ALCOHOL SPECTRUM DISORDER (FASD) is complex and
guidelines are warranted. A subcommittee of the Public Health Agency
of Canada's National Advisory Committee on Fetal Alcohol Spectrum
Disorder reviewed, analysed and integrated current approaches to
diagnosis to reach agreement on a standard in Canada. The purpose of
this paper is to review and clarify the use of current diagnostic
systems and make recommendations on their application for diagnosis
of FASD-related disabilities in people of all ages. The guidelines
are based on widespread consultation of expert practitioners and
partners in the field. The guidelines have been organized into 7
categories: screening and referral; the physical examination and
differential diagnosis; the neurobehavioural assessment; and
treatment and follow-up; maternal alcohol history in pregnancy;
diagnostic criteria for fetal alcohol syndrome (FAS), partial FAS and
alcohol-related neurodevelopmental disorder; and harmonization of
Institute of Medicine and 4-Digit Diagnostic Code approaches. The
diagnosis requires a comprehensive history and physical and
neurobehavioural assessments; a multidisciplinary approach is
necessary. These are the first Canadian guidelines for the diagnosis
of FAS and its related disabilities, developed by broad-based
consultation among experts in diagnosis.
In this document, we discuss the diagnostic approach to disabilities
associated with prenatal alcohol exposure. Fetal alcohol spectrum
disorder (FASD), along with its most visible presentation, fetal
alcohol syndrome (FAS), is a serious health and social concern
to Canadians. FASD is an umbrella term describing the range of
effects that can occur in an individual whose mother drank alcohol
during pregnancy. These effects may include physical, mental,
behavioural and learning disabilities with lifelong implications. The
term FASD is not intended for use as a clinical diagnosis.
FASD is the result of maternal alcohol consumption during pregnancy
and has implications for the affected person, the mother, the
family and the community. Since FAS was first described in 1973,1
it has become apparent that it is complex; affected people exhibit
a wide range of expression, from severe growth restriction,
intellectual disability, birth defects and characteristic dysmorphic
facial features to normal growth, facial features and intellectual
abilities, but with lifelong deficits in several domains of
brain function. FASD requires a medical diagnosis in the context
of a multidisciplinary assessment. FASD itself is not a diagnostic
term. The purpose of this paper is to review and clarify the
use of the current diagnostic systems and make recommendations
on their application for diagnosis of FASD-related disabilities
in people of all ages. For a description of the characteristics
and the natural course of FASD, consult some of the broader
reviews.2,3,4,5,6,7
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Epidemiology of FASD
|
The
prevalence of FAS in the United States has been reported as 1–3 per
1000 live births and the rate of FASD as 9.1 per 1000 live
births.8,9,10,11
However, diagnosis may often be delayed or missed entirely.2
There are no national statistics on the rates of FASD in Canada, although
studies have estimated its prevalence in small populations. In an
isolated Aboriginal community in British Columbia, FASD prevalence
was 190 per 1000 live births.12 In
northeastern Manitoba, an incidence of about 7.2 per 1000 live births
was found.13
In another Manitoba study in a First Nations community,14 the
prevalence of FAS and partial FAS was estimated to be 55–101 per
1000. In their survey, Asante and Nelms-Matzke15 estimated
the rate of FAS and related effects at 46 per 1000 native Canadian
children in the Yukon and 25 per 1000 in northern British Columbia.
Based on referrals to a diagnostic clinic in Saskatchewan, the rate
of FAS was estimated at 0.589 per 1000 live births in 1988–1992 and
0.515 per 1000 in 1973–1977.16 However,
none of these data should be generalized to other communities, other
populations or the Canadian population in general.
| FASlink Editor's Note: The above studies
were conducted as many as 25 years before the diagnostic criteria for Canada
were finally set and were all of remote, small First Nation's communities.
First Nations comprise 2.2% of the Canadian population. More recent statistical
analysis of the Statistics Canada - Canadian Community Health Survey, Binge
Drinking studies, combined with birth and population statistics, indicate
79% of Canadian children are exposed to alcohol during pregnancy, including
37% who are exposed to binge drinking (5+ drinks per occasion, multiple
times). Meconium assay testing of newborns for FAEE's formed by maternal
alcohol consumption indicate 15% to 18% have been exposed to alcohol in
the final 20 weeks of pregnancy, including 4% at elevated levels. In an
Ontario 28,000 student population, 21.4% are receiving Special Education
services, most for conditions of types known to be caused by prenatal exposure
to alcohol. |
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Risk factors
|
A
common misconception is that FASD is associated with ethnocultural
background. However, the data suggest that risk factors for
prenatal alcohol exposure include higher maternal age and lower
education level, prenatal exposure to cocaine and smoking, custody
changes, lower socioeconomic status and paternal drinking and
drug use at the time of pregnancy;17 and
reduced access to prenatal and postnatal care and services,
inadequate nutrition and a poor developmental environment (e.g.,
stress, abuse, neglect).18
In a 5-year follow-up study of birth mothers of children with full
FAS, Astley and colleagues19
found that these women came from diverse racial, educational and
economic backgrounds. They were often challenged by untreated or
under-treated mental health concerns, they were socially isolated,
they were victims of abuse and they had histories of severe childhood
sexual abuse.
Because there are no large-scale studies of risk factors and
because risks are interrelated and could be different for different
populations, it is difficult to provide accurate figures for
relative risk. However, the most important risk factor for FASD
is related to high blood-alcohol concentration: the timing of
exposure during fetal development, the pattern of consumption,
i.e., binge drinking (4 or more drinks per occasion) and the
frequency of use. Although there seems to be no definite threshold
of exposure, there appears to be a dose-response relation.17,20,21
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Importance of early
diagnosis |
An early
diagnosis is essential to allow access to interventions and resources
that may mitigate the development of subsequent "secondary
disabilities" (e.g., unemployment, mental health problems, trouble
with the law, inappropriate sexual behaviour, disrupted school
experience) among affected people.22
Furthermore, an early diagnosis will also allow appropriate
intervention, counselling and treatment for the mother and may
prevent the birth of affected children in the future.23 It
may also prompt caregivers to seek diagnosis and support for
previously undiagnosed siblings. A review of medical and behavioural
management of those with FASD can be found in other sources.3,24
Astley and Clarren25
suggest that accurate and timely diagnosis is essential to improve
outcome, as misclassification leads to inappropriate patient care,
increased risk of secondary disabilities, missed opportunities for
prevention and inaccurate estimates of incidence and prevalence.
Together, these inaccuracies could hinder efforts to allocate
sufficient social and health care services to the vulnerable
populations and preclude accurate assessment of primary prevention
efforts.
Because of limited capacity and expertise and the need to involve
several professionals in a comprehensive multidisciplinary diagnostic
evaluation, only a fraction of those affected currently receive
a diagnosis. Results26
from the Canadian national survey regarding knowledge and attitudes
of health professionals suggest that standardized guidelines for
diagnosis and further professional education and training are needed
for practitioners to participate in diagnosis. In response to these
concerns, Health Canada's National Advisory Committee on FASD, along
with experts and practitioners in FAS diagnosis and treatment,
present the following guidelines for diagnosis.
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Process of guideline
development |
These
guidelines are the result of more than 10 face-to-face consultations
with Canadian and American experts in the diagnosis of FAS and its
related disabilities (Appendix
1). Many of the participants are currently providing diagnostic
services across Canada. Review and feedback were provided by a
diverse group of individuals; professional organizations and
societies; and provincial, territorial and federal levels of
government. Guidelines are presented in 6 areas related to the
diagnostic process: 1. screening and referral; 2. the physical
examination and differential diagnosis; 3. neurobehavioural
assessment; 4. treatment and follow-up; 5. maternal alcohol history
in pregnancy; and 6. diagnostic criteria for FAS, partial FAS and
alcohol-related neurodevelopmental disorder. We also include
recommendations for harmonization of the 2 main approaches to
diagnosis.
There are multiple approaches to diagnosis, and the working group
sought to integrate these to achieve consistent diagnoses across
Canada. Current knowledge of the complexity of the disabilities
associated with prenatal alcohol exposure dictates that a
comprehensive, multidisciplinary assessment is necessary to make an
accurate diagnosis and provide recommendations for management. We
are recommending such a multidisciplinary approach. This
approach will also allow for collection of Canadian data for
estimating incidence and prevalence of FASD. This information is
essential to identify the need for and the development of
appropriate prevention and intervention programs and services.
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Background and
terminology for the diagnosis of FAS |
The first
recognition of a variety of birth defects and developmental
disabilities in offspring born to alcoholic parents is attributed
to Lemoine and colleagues.27 A
specific pattern of birth defects following maternal alcohol exposure
was described in the United States.1,28 The
specific pattern, referred to as FAS, consists of facial
abnormalities (smooth philtrum [the space between the upper lip and
the nose], thin vermilion border [the exposed mucosal, or red part,
of the upper lip], short palpebral fissures), impaired prenatal or
postnatal growth (or both) and central nervous system or
neurobehavioural disorders. Alcohol probably acts through multiple
mechanisms and a range of disabilities has been observed in the
absence of dysmorphic features reflecting varying degrees of damage
during fetal development; undoubtedly, timing and degree of exposure
are important variables that contribute to the variation. Thus, the
term "suspected fetal alcohol effects" (FAE) was created.29
These "effects" were further delineated by the United States'
Institute of Medicine (IOM), which published recommendations in 1996
for diagnosis of FAS in consultation with a panel of experts.4 The
diagnostic categories presented were: FAS with and without a
confirmed history of alcohol exposure, partial FAS, alcohol-related
birth defects (ARBD), and alcohol-related neurodevelopmental disorder
(ARND) (Table
1).
In
the late 1990s, another diagnostic strategy was developed by Astley
and Clarren.25,30They
created a 4-Digit Diagnostic Code using data from the Washington
State Fetal Alcohol Syndrome Diagnostic and Prevention Network of
clinics. The system uses quantitative, objective measurement scales
and specific case definitions. The 4 digits in the code reflect the
magnitude of expression or severity of the 4 key diagnostic features
of FAS in the following order: growth deficiency; the FAS facial
phenotype; central nervous system damage or dysfunction; gestational
exposure to alcohol. The magnitude of expression of each feature
is ranked independently on a 4-point Likert scale with 1 reflecting
complete absence of the feature and 4 reflecting its extreme
expression. The 4-Digit Diagnostic Code is now being used for
diagnosis, screening and surveillance in clinics throughout the
United States and Canada. Terminology from Astley's 2004 revision of
the 4-Digit Diagnostic Code are used in this article.*
Although the approaches are different, the underlying, fundamental
criteria of the IOM and the 4-Digit Diagnostic Code are similar.
Some clinics are choosing to integrate the diagnostic tools and
precision reflected in the 4-Digit Diagnostic Code with the
diagnostic categories and language recommended by the IOM committee.
Although both IOM criteria and the 4-Digit Diagnostic Code have been
published, many clinicians still use the less desirable and
potentially misleading gestalt approach (Table 2).
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
The diagnostic process
|
The
diagnostic process consists of screening and referral, the physical
examination and differential diagnosis, the neurobehavioural
assessment and treatment and follow-up. Because of the complexity
and the range of expression of dysfunction related to prenatal
alcohol exposure, a multidisciplinary team is essential for an
accurate and comprehensive diagnosis and treatment recommendations.
The assessment process begins with recognition of the need for
diagnosis and ends with implementation of appropriate
recommendations. The multidisciplinary diagnostic team can be
geographic, regional or virtual; it can also accept referrals from
distant communities and carry out an evaluation using
telemedicine.
The core team may vary according to the specific context, but
ideally it should consist of the following professionals with
appropriate qualifications, training and experience in their
particular discipline:
- Coordinator for case management (e.g., nurse, social worker).
- Physician specifically trained in FASD diagnosis.
- Psychologist.
- Occupational therapist.
- Speech-language pathologist.
Additional members may include addiction counsellors, childcare
workers, cultural interpreters, mental health workers, parents
or caregivers, probation officers, psychiatrists, teachers,
vocational counselors, nurses, geneticists or dysmorphologists,
neuropsychologists, family therapists.
Comments
Clearly, funding for development, training and maintenance of
multidisciplinary diagnostic teams is necessary so that major
centres will have the expertise and capacity to serve their
communities. To optimize the outcome of the diagnosis, the community
and the family must be prepared, ready to participate in, and
be in agreement with the diagnostic assessment. The diagnostic
process should be sensitive to the family's and the caregiver's
needs. In each community, referrals must be evaluated and their
level of priority established. The family and guardian must be
in agreement on the purpose of diagnosis. They must be made aware of
the potential psychosocial consequences of a diagnosis of FASD (e.g.,
increasing a sense of guilt and anger, especially with the birth
mother, or potential stigmatization of the child). The family or
guardian will likely need help to move confidently through the
diagnostic process. This help might include some preparatory
education concerning FASD and linking them with community supports
and resources.
Information from multiple sources (e.g., school records, hospital
records, social services, previous assessments) should be obtained;
this might involve meetings with relevant professionals who
know the patient (e.g., teachers, physicians, social workers,
psychologists). Other relevant documentation would include birth
and pregnancy records, medical and hospital records, adoption
records, academic records, achievement tests, developmental
assessments, psychological and psychometric assessments, legal
reports and documentation of the family history.
The comprehensive assessment by the diagnostic team provides
important information about the individual's unique needs and
allows interventions to be tailored to his or her strengths and
challenges. The post-diagnostic report should state the basis for the
diagnosis by including the history of alcohol use, the physical
criteria and the psychological data that support it.
Multidisciplinary teams work with community partners and resources
to develop and implement management plans to maximize the potential
of the affected individual. Following assessment, a report containing
recommendations should be made available to caregivers, educators,
and biological families, as well as other appropriate individuals
who work with the child (i.e., daycare workers, early intervention
workers, social workers, etc). The team findings should be discussed
with the guardian. Older children who have the cognitive ability
should have the opportunity to learn about their diagnosis from
the team. The team might also take on the responsibility for
facilitating and providing follow-up with the family and community
resources regarding outcomes of the recommendations. Ultimately,
the diagnostic process will result in concrete management
recommendations to improve the lives of the affected individuals,
their families and the communities.
Canada is a large country with vast distances between communities,
some of which are remote and isolated. Specialists providing
consultation to remote areas require specialized training in
FASD assessment and need to link with centres that have
multidisciplinary teams to assist in the diagnostic process. A number
of tools may be useful for distant diagnosis. More frequent use of
telemedicine, for example, will allow assessment of children in
distant communities.31Other
examples include the use of digital photographs32,33 and
3-D laser surface scanning34,35sent
electronically to teams in larger centres.
We recognize that there is currently a limited capacity even in
some large communities in Canada to provide a multidisciplinary
team-based approach to FAS diagnosis. Professionals should make
the best use of available resources and expertise to provide an
accurate assessment and treatment plan for affected individuals and
their families, recognizing the key role of psychology.
1. Screening and
referral
Recommendations
- 1.1 All pregnant and post-partum women should be screened
for alcohol use with validated screening tools (i.e.,
T-ACE, TWEAK) by relevant health care providers. Women at
risk for heavy alcohol use should receive early brief
intervention (i.e., counselling).
- 1.2 Abstinence should be recommended to all women during
pregnancy, as the mother's continued drinking during
pregnancy will put the fetus at risk for effects related
to prenatal alcohol exposure.
- 1.3 Referral of individuals for a possible FASD-related
diagnosis should be made in the following
situations:
- Presence of 3 characteristic facial features (short
palpebral fissures, smooth or flattened philtrum, thin
vermilion border).
- Evidence of significant prenatal exposure to alcohol at
levels known to be associated with physical or
developmental effects, or both.
- Presence of 1 or more facial features with growth
deficits plus known or probable significant
prenatal alcohol exposure.
- Presence of 1 or more facial features with 1 or
more central nervous system deficits plus known or
probable significant prenatal alcohol
exposure.
- Presence of 1 or more facial features with pre- or
postnatal growth deficits, or both (at the
10th percentile or below [1.5 standard deviations
below the mean]) and 1 or more central nervous
system deficits plus known or probable
significant prenatal alcohol exposure.
- 1.4 Individuals with learning or behavioural difficulties,
or both, without physical or dysmorphic features and
without known or likely prenatal alcohol exposure should
be assessed by appropriate professionals or specialty
clinics (i.e., developmental pediatrics, clinical
genetics, psychiatry, psychology) to identify and treat
their problems.
Comments
- Screening should not be equated with diagnosis. We know
that in some places with no diagnostic services, screening
tools have been inappropriately used in lieu of a proper
diagnosis. One purpose of screening is to identify and refer
pregnant women who may be at risk for an alcohol use disorder and
who may place their child at risk for FASD. Several alcohol
screening tools have been found to be effective in identifying
problem drinking in a primary care setting (e.g., TWEAK, T-ACE,
CAGE, AUDIT, S-MAST, B-MAST).2,36,37,38
- There is moderate evidence37,38
to support the use of T-ACE and TWEAK to identify women
who would benefit from intervention for alcohol use
during pregnancy. If the woman cannot abstain, she
should receive support and be referred to appropriate
counselling and treatment. Stopping drinking at any
point during the pregnancy will improve the outcome for
the baby. Research is being carried out to develop
gender and culturally appropriate instruments for the
screening of all women during their child-bearing years.38
- The purpose of screening individuals at risk for the
effects of prenatal alcohol exposure is to determine
whether a pattern of learning and behavioural problems
may be related to prenatal alcohol exposure. The
screening could be conducted through the education
system, the mental health system, the judicial system or
social services. The purpose of screening should be to
facilitate referral to a diagnostic clinic and highlight
the need for referral and support for the birth
mother.
- The FAS Diagnostic and Prevention Network has had
encouraging results in applying the FAS facial
photographic screening tool in foster children and
school-age children populations.39
However, in the wide array of FASDs, facial
dysmorphology is often absent and, in the final analysis,
has little importance compared with the impact of prenatal
alcohol exposure on brain function. However, it is
important to note that the facial phenotype is a midline
defect that is the most sensitive and specific marker for
alcohol-related brain damage.
- All those suspected of having brain dysfunction should be
referred to an appropriate professional or clinic for
assessment (i.e., developmental pediatrics, clinical
genetics, psychiatry, psychology). Because of the
specificity of FASD clinics in addressing issues related
to prenatal alcohol exposure, those with no prenatal alcohol
exposure should be referred to an appropriate professional or
clinic for assessment, treatment and follow-up.
2. The physical examination and
differential diagnosis
The purpose of dysmorphology assessment is to identify those with
features related to prenatal alcohol exposure and also to identify
children with dysmorphic features due to other causes. Occasionally,
children with prenatal alcohol effects may have another genetic
syndrome as a comorbidity. When in doubt and if feasible, a genetic
dysmorphology assesment is advisable.
A general physical and neurologic examination, including appropriate
measurements of growth and head size, assessment of characteristic
findings and documentation of anomalies (e.g., cleft palate,
congenital heart defects, epicanthic folds, high arched palate,
poorly aligned or abnormal teeth, hypertelorism, micrognathia,
abnormal hair patterning, abnormal palmar creases, skin lesions)
is required to exclude the presence of other genetic disorders
or multifactorial disorders that could lead to features mimicking
FAS or partial FAS (Table 3).
Some children will have significant neurologic deficits, such as
deafness, blindness or seizures, and these should be assessed and
documented as essential components of the child's profile. These
features do not discriminate alcohol-exposed from unexposed children.
The face of FAS is the result of a specific effect of ethanol
teratogenesis altering growth of the midface and brain. Those exposed
to other embryotoxic agents may display a similar, but not identical,
phenotypic facial development, impaired growth, a higher frequency of
anomalies and developmental and behavioural abnormalities (for a
review, see Chudley and Longstaffe24).
However, because FAS facial criteria have been restricted to short
palpebral fissures, smooth philtrum and thin upper lip, there is far
less overlap with the facial phenotypes associated with other
syndromes. Knowledge of exposure history will decrease the
possibility of misdiagnosing FASD.
Children may be found to need other medical assessments to address
co-occurring issues. For example, sleep disturbance is common
with prenatal alcohol exposure and medical problems related to
obstructive sleep apnea may have been overlooked previously. Atypical
seizures may also be present and endocrinopathies may exist as a
comorbid reason for growth deficiency. These individuals should be
assessed by appropriate health professionals.
2a. Growth
Recommendations
- 2.1 Growth should be monitored to detect deficiency.
Presence of pre- or post-natal growth deficiency, defined
as height or weight at or below the 10th percentile (1.5
standard deviations below the mean) or a
disproportionately low weight-to-height ratio (at or below the 10th
percentile) using appropriate norms. To determine that a child is
growth deficient requires taking into consideration confounding
variables such as parental size, genetic potential and associated
conditions (e.g., gestational diabetes, nutritional status,
illness).
Comments
- Children affected by prenatal alcohol exposure may have
prenatal or postnatal growth deficits. They can be small
for gestational age in utero and remain below average
throughout their lives with respect to head
circumference, weight and height. Many children can have
normal growth parameters, but be at risk in later
development for clinically significant learning, behavioural and
cognitive deficits. If there is no alcohol exposure in the third
trimester, the growth parameters can be normal. Gestational
diabetes can lead to increased fetal size, which can mask the
effects of growth retardation from prenatal alcohol exposure.
Furthermore, if the infant is born into a family or a community
where "normal" size is above the average for the general
population, growth impairment may be masked if the child is
compared with standard growth parameters rather than community
norms.14
Growth deficiencies may not persist with age, and infant growth
records may not be available for adults coming in for assessment
for the first time. There is a need to establish growth norms
for the Canadian population and subpopulations that differ
from the general population.
2b. Facial
features
Recommendations
- 2.2 The 3 characteristic facial features that discriminate
individuals with and without FAS are:
- Short palpebral fissures, at or below the 3rd percentile
(2 standard deviations below the mean).
- Smooth or flattened philtrum, 4 or 5 on the
5-point Likert scale of the lip-philtrum guide.25,39
- Thin vermilion border of the upper lip, 4 or 5 on the
5-point Likert scale of the lip-philtrum guide.
- 2.3 Associated physical features (abnormalities such as
midface hypoplasia, micrognathia, abnormal position or
formation of the ears, high arched palate,
hypertelorism, epicanthic folds, limb and palmar crease
abnormalities and short-upturned nose) should be
recorded but do not contribute to establishing the
diagnosis.
- 2.4 Facial features should be measured in all age groups.
If a patient's facial features change with age, the
diagnosis of the facial features should be based on the
point in time when the features were most severely
expressed. When diagnosing adults, it can be helpful to
view childhood photographs.
Comments
- A characteristic craniofacial profile associated with FAS
was first described by Jones and Smith40
in 1975 and later refined by Astley, Clarren and
others.25,32,39
Individuals with FAS have short palpebral fissures, a
thin upper lip and an indistinct philtrum (Fig. 1).
Palpebral fissure length, philtrum and upper lip differ with race
and age. Growth and facial anthropometric data are needed for the
specific population, as sensitivity and specificity of the
assessment will be lowered without the use of appropriate norms.
Some discriminating characteristic features in FAS (i.e., upper lip
or philtrum) may become less recognizable with age, making accurate
diagnosis more difficult in older groups, but facial features
should always be measured. More longitudinal research is needed to
correlate changes in these characteristic physical findings in
adolescents and adults diagnosed with FAS or partial FAS.
Palpebral fissure length (Fig. 2) is
difficult to measure accurately without training. Thomas
and co-workers41
have published norms for palpebral fissure length at 29
weeks gestation to 14 years. There are a number of
opinions about which norms are appropriate,41,42,43,44
but it is generally agreed that all are flawed in some
respect.
Two graphs of palpebral fissure length
are presented in Appendix 2. Some discrepancies exist.
Both studies used North American white subjects;
standards for other populations in Canada are not
currently available. Appendix
2-1 may be more reliable when measuring palpebral
fissure length using a plastic ruler (in the experience
of one of the authors); Appendix
2-2 may be more reliable if slide calipers are used
(in the experience of one of the authors). Percentile
ranks for both graphs seem to be in agreement until age
7 years, after which Appendix 2-2 shows longer palpebral
fissures in older children and adolescents than Appendix 2-1. We
believe this may be due to differences in measurement technique.
Because calipers are not a common tool in most medical clinics, we
recommend the use of a clear flexible plastic ruler.
There is a need to establish updated norms
for all ages and subpopulations. Astley and Clarren25,39
have developed norms for the assessment of the lip and
philtrum using their pictorial guide. Lip-philtrum
guides were developed for use in Caucasian and
African-American populations, but no standards are currently
available for other populations.
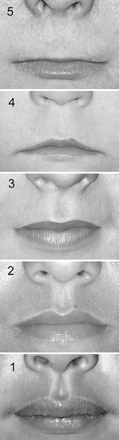
View larger
version (49K):
[in
this window]
[in a new window]
|
Fig. 1: Lip-philtrum guide. A
5-point pictorial scale for measuring philtrum smoothness and upper
lip thinness. Features are measured independently; for example, an
individual can have a rank 5 philtrum and a rank 1 upper lip. Photo:
Susan Astley, FAS
DPN
| |
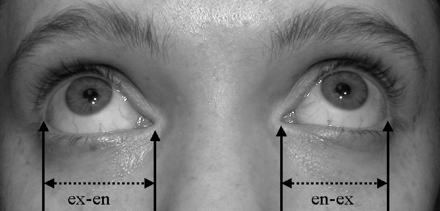
View larger
version (87K):
[in
this window]
[in a new window]
|
Fig. 2: Palpebral fissure length.
To measure palpebral fissure length, identify the inner corner or
encanthion (en) and outer corner or excanthion (ex) for each eye.
Have the patient look up so that ex can be seen clearly. With a
clear flexible ruler held in the horizontal plane, measure the
length of each ex-en interval immediately below the eye, being
careful not to touch the eye or eyelashes. Plot the result on an
appropriate nomogram chart to determine the percentile or standard
deviation for each eye. Photo: Albert
Chudley
| |
3. Neurobehavioural
assessment
Recommendations
- 3.1 The following domains should be assessed:
- Hard and soft neurologic signs (including sensory-motor signs).
- Brain structure (occipitofrontal circumference, magnetic resonance
imaging, etc.).
- Cognition (IQ).
- Communication: receptive and expressive.
- Academic achievement.
- Memory.
- Executive functioning and abstract reasoning.
- Attention deficit/hyperactivity.
- Adaptive behaviour, social skills, social communication.
- 3.2 The assessment should include and compare basic and complex
tasks in each domain, as appropriate.
- 3.3 The domains should be assessed as though they were
independent entities, but where there is overlap
experienced clinical judgment is required to decide how
many domains are affected.
- 3.4 A domain is considered "impaired" when on a
standardized measure:
- Scores are 2 standard deviations or more below the mean,
or
- There is a discrepancy of at least 1 standard deviation
between subdomains. For example:
- i. Verbal v. non-verbal ability on standard IQ tests,
- ii. Expressive v. receptive language,
- iii. Verbal v. visual memory, or
- There is a discrepancy of at least 1.5–2 standard
deviations among subtests on a measure, taking
into account the reliability of the specific
measure and normal variability in the population.
- 3.5 In areas where standardized measurements are not
available, a clinical judgment of "significant dysfunction"
is made, taking into consideration that important
variables, including the child's age, mental health
factors, socioeconomic factors and disrupted family or
home environment (e.g., multiple foster placements,
history of abuse and neglect), may affect development but
do not indicate brain damage.
- 3.6 Evidence of impairment in 3 domains is necessary for a
diagnosis, but a comprehensive assessment requires that
each domain be assessed to identify strengths and
weaknesses.
- 3.7 The diagnosis should be deferred for some at-risk
children (e.g., preschool-age) who have been exposed to
alcohol but may not yet demonstrate measurable deficits
in the brain domains or may be too young to be tested in
all the domains. However, developmental assessment
should identify areas for early intervention.
Examples of tests that are most widely used to assess the domains
and their criteria are provided in Appendix
3.
Comments
- Research reports have documented a range of cognitive and
behavioural outcomes associated with prenatal alcohol
exposure. Contemporary studies have reported some of
these outcomes in the absence of FAS physical features.
Currently, no modal profile of abilities has been found
to be unique to alcohol exposure, is observed in all
those with prenatal alcohol exposure, or can be distinguished
from that observed with some other neurobehavioural
disorders. Furthermore, not every deficit that we may
identify in a child with prenatal exposure to alcohol
may be solely the result of alcohol exposure. An expert
analysis of neurodevelopmental deficits caused by a
range of teratogens and congenital disorders failed to
result in a consensus on core deficits associated only with
FASD.4
- Research and experience has shown that features of FASD
are complex and multifaceted, originating with organic
brain damage caused by alcohol, but interacting with
genetic and other influences. Over the lifespan of the
affected person, these features may be exacerbated or
mitigated by environmental experiences.
- To make the diagnosis of FAS, features such as
microcephaly, structural abnormalities (as may be detected
on brain scans) and hard neurologic signs are taken as
strong evidence of organic brain damage. We believe that
low-average to borderline intelligence and soft
neurologic signs alone are insufficient evidence of
brain damage because they are frequently found in the
general population. Features such as learning difficulties,
attention deficit/hyperactivity disorder and deficits in
adaptive skills, memory, higher-level language and
abstract thinking are frequently seen in children with
prenatal alcohol exposure, but also among those with
other etiologies. These deficits can be multifactorial
in etiology and can also be attributed to genetics or
postnatal experiences.
- The 4-Digit Diagnostic Code evaluation of the FASD brain
is based on levels of certainty, in the judgement of the
clinician, that the individual's cognitive and
behavioural problems reflect brain damage. A higher rating
may reflect a more severe expression of functional
disability, asynchronous patterns across domains or
certainty based on deficits in multiple domains. The
determination is based on objective evidence of
"substantial deficiencies or discrepancies across
multiple areas of brain performance."25,39
- The IOM4
also requires "evidence of a complex pattern of behavior
or cognitive abnormalities that are inconsistent with
developmental level and cannot be explained by familial
background or environment alone, such as learning difficulties;
deficits in school performance; poor impulse control; problems in
social perception; deficits in higher level receptive and
expressive language; poor capacity for abstraction or
metacognition; specific deficits in mathematical skills; or
problems in memory, attention, or judgment," but is much less
specific than the 4-Digit Diagnostic Code with regard to the
criteria for determining the deficit.
- We have adapted the method of the 4-Digit Diagnostic Code
with regard to identifying domains and severity of impairment
or certainty of brain damage. Current research shows overlap
between the neurobehavioural outcomes in FAS and ARND diagnostic
groups when neuropsychologic data are compared.45
In addition, we believe that a single feature such as microcephaly
is not a sufficient indicator of brain damage for the purposes of
an FAS diagnosis because it may reflect genetic or ethnic
differences not reflected in currently available physical norms.
Our concern is that there may be an over-diagnosis of FAS if
evidence of brain damage is based on a single indicator as allowed
by both the 4-Digit Diagnostic Code and the IOM models. An
individual showing hard neurologic signs or structural brain
abnormalities (i.e., true brain damage) will likely show additional
functional deficits in the listed domains. A diagnosis of full FAS
will not be denied by combining the criteria for full FAS and ARND
in this harmonized system.
- Although the domains are considered to be separate and
independent entities, there is obviously overlap. For example,
a discrepancy between verbal and non-verbal scores on an
IQ test (taking into account normal variability in the
population) may be reflecting a specific language
disability. If language is deficient, can deficits in
verbal memory be considered an additional domain? Does a
language deficit represent brain damage if the child has
experienced a prolonged period of social deprivation? The
cut-off of 2 standard deviations below the mean on standardized
tests is recommended to increase confidence that abilities in
the domain are impaired as a result of brain damage and are
scored as "3" (significant dysfunction) on the 4-Digit Diagnostic
Code. With 3 such domains, the brain rank is 3: "probable brain
dysfunction."
- We realize that in standard neuropsychologic practice, 1.5
standard deviations below the mean may indicate subtle
impairments. Using the 4-Digit Diagnostic Code, the domains
would be scored as "moderate dysfunction" and may result
in a brain rank of 2: "possible brain dysfunction."
These more subtle findings are an important part of the
individual's profile. For the purpose of diagnosis,
however, and the certainty that the scores represent
injury caused by alcohol, the more extreme cut-off is
recommended. The multidisciplinary team, reviewing the
data and using experienced clinical judgement, is critical
in making an accurate diagnosis as qualitative aspects of
performance are also important. The diagnostic profile
is dynamic and may change over time; thus individuals
affected or suspected to be affected may require several
assessments over time. Services should not be based on
the diagnosis itself, but rather on the profile of brain
function-dysfunction.
4. Treatment and
follow-up
Recommendations
- 4.1 Education of the patient and family members on
features of FASD is crucial. The potential psychosocial
tensions that might be expected to develop within the
family as a result of the diagnosis should also be
discussed. This must be done in a culturally sensitive
manner using appropriate language.
- 4.2 A member of the diagnostic team should follow-up outcomes
of diagnostic assessments and treatment plans within a reasonable
length of time to assure that the recommendations have been
addressed.
- 4.3 Diagnosed individuals and their families should be
linked to resources and services that will improve outcome.
However, where services are limited in the community, an
individual should not be denied an assessment for
diagnosis and treatment. Often the diagnosis in the
individual is the impetus that leads to the development
of resources.
5. Maternal alcohol history in
pregnancy
Recommendations
- 5.1 Prenatal alcohol exposure requires confirmation of
alcohol consumption by the mother during the index pregnancy
based on reliable clinical observation, self-report,
reports by a reliable source or medical records
documenting positive blood alcohol, alcohol treatment or
other social, legal or medical problems related to
drinking during the pregnancy.
- 5.2 The number and type(s) of alcoholic beverages consumed
(dose), the pattern of drinking and the frequency of
drinking should all be documented if available.
- 5.3 Hearsay, lifestyle, other drug use or history of
alcohol exposure in previous pregnancies cannot, in
isolation, be informative of drinking patterns in the
index pregnancy. However, co-occurring disorders, significant
psychosocial stressors and prenatal exposure to other
substances (e.g., smoking, licit or illicit drugs) in
the index and previous pregnancies should still be recorded,
based on known interactive effects of these variables on
the severity of pregnancy outcomes for both the mother
and her offspring.
Comments
- Gathering reliable information about maternal drinking is
key to establishing an accurate diagnosis. Special attention
must be paid to inquiring about maternal alcohol use
before the woman recognized that she was pregnant. Some
women do not consider that their prior drinking is
important and many underreport it. Training is required in how to
obtain this information in a non-threatening, non- judgmental
way.
- Canadian survey data suggest that the number of women who
report drinking during pregnancy has decreased. The
National Population Health Survey,
1994–199546
and National Longitudinal Survey of Children and
Youth, 1994–199547
reported that 17–25% of women drank alcohol at some
point during their pregnancy and 7–9% drank alcohol
throughout their pregnancy. According to the National
Longitudinal Survey of Children and Youth, 1998–199948
14.4% of women drank at some point during their pregnancy and
4.9% drank throughout their entire pregnancy (3% reported binge
drinking during pregnancy). In the Fall 2002 Survey of First
Nations People Living on Reserve,49
53% of the respondents said that cutting down or stopping alcohol
use was important for women to have a healthy baby.
- The evaluation of "significant alcohol exposure" is often
confusing. The IOM describes significant alcohol
exposure as "a pattern of excessive intake characterized
by substantial, regular intake or heavy episodic
drinking"4
(the National Institute on Alcohol, Alcoholism, and
Alcohol Abuse defines heavy alcohol use as drinking 5 or
more drinks per occasion on 5 or more days in the past
30 days36).
Evidence of this pattern may include frequent episodes
of intoxication, development of tolerance or withdrawal,
social problems related to drinking, legal problems
related to drinking, engaging in physically hazardous
behaviour while drinking, or alcohol-related medical
problems such as hepatic disease. As further research is
completed and as, or if, lower quantities or variable patterns
of alcohol use are associated with alcohol-related birth
defects (ARBD) or ARND, these patterns of alcohol use
should be incorporated into the diagnostic criteria.4
6. Diagnostic criteria for FAS,
partial FAS and ARND
Recommendations
- 6.1 The criteria for the diagnosis of fetal alcohol
syndrome, after excluding other diagnoses, are:
- Evidence of prenatal or postnatal growth impairment, as in
at least 1 of the following:
- Birth weight or birth length at or below the
10th percentile for gestational age.
- Height or weight at or below the 10th
percentile for age.
- Disproportionately low weight-to-height
ratio (= 10th percentile).
- Simultaneous presentation of all 3 of the following facial
anomalies at any age:
- Short palpebral fissure length (2 or more standard deviations
below the mean).
- Smooth or flattened philtrum (rank 4 or 5 on the
lip-philtrum guide).
- Thin upper lip (rank 4 or 5 on the lip-philtrum
guide).
- Evidence of impairment in 3 or more of the following
central nervous system domains: hard and soft
neurologic signs; brain structure; cognition; communication;
academic achievement; memory; executive functioning and
abstract reasoning; attention deficit/hyperactivity;
adaptive behaviour, social skills, social
communication.
- Confirmed (or unconfirmed) maternal alcohol exposure.
- 6.2 The diagnostic criteria for partial fetal alcohol
syndrome, after excluding other diagnoses, are:
- Simultaneous presentation of 2 of the following facial
anomalies at any age:
- Short palpebral fissure length (2 or more standard
deviations below the mean).
- Smooth or flattened philtrum (rank 4 or 5
on the lip-philtrum guide).
- Thin upper lip (rank 4 or 5 on the
lip-philtrum guide).
- Evidence of impairment in 3 or more of the
following central nervous system domains:
hard and soft neurologic signs; brain structure;
cognition; communication; academic achievement; memory;
executive functioning and abstract reasoning; attention
deficit/hyperactivity; adaptive behaviour, social
skills, social communication.
- Confirmed maternal alcohol exposure.
- 6.3 The diagnostic criteria for alcohol-related
neurodevelopmental disorder, after excluding other
diagnoses, are:
- Evidence of impairment in 3 or more of the following
central nervous system domains: hard and soft
neurologic signs; brain structure; cognition;
communication; academic achievement; memory; executive
functioning and abstract reasoning; attention
deficit/hyperactivity; adaptive behaviour, social
skills, social communication.
- Confirmed maternal alcohol exposure.
- 6.4 The term alcohol-related birth defects (ARBD) should
not be used as an umbrella or diagnostic term, for the
spectrum of alcohol effects. ARBD constitutes a list of
congenital anomalies, including malformations and
dysplasias and should be used with caution (Table
1).
Comments
- Our definition of partial FAS differs from the published
IOM criteria.4
Where significant prenatal alcohol exposure is known and
there is significant growth retardation and significant indicative
facial features but no evidence of brain involvement, a diagnosis
of partial FAS could be made using the IOM criteria. It is our view
that, using the term partial FAS in the absence of measurable brain
deficits could be harmful for the individual because the diagnosis
of partial FAS implies brain dysfunction. If some characteristic
facial features and growth impairment, without significant
developmental or behavioural problems, are found in children under
6 years of age, it would be prudent to say that the child may be at
risk of learning and behaviour problems at a later time due to
prenatal alcohol exposure. No alcohol-related diagnosis should be
made, but the child must be monitored by the family physician or
health care worker and deficits should be documented using a
neurodevelopmental assessment.
- The term "partial" in partial FAS does not imply that these
individuals are less severely impaired in day-to-day functioning
than those with a diagnosis of FAS, as the deficits in brain
function may be similar.
7. Harmonization of the Institute
of Medicine (IOM) and 4-Digit Diagnostic Code approaches
Recommendations
- 7.1 The approach identified in the 4-Digit Diagnostic Code
should be used to describe, assess and measure objectively
alcohol exposure, growth, facial features and brain
damage. The 4-Digit Diagnostic Code should be recorded
for each assessment and may be useful for surveillance
and research purposes.
- 7.2 The terminology in the IOM criteria should be used to
describe the diagnosis.
Comments
- Table
4 and Table 5
illustrate how we recommend harmonizing the IOM and 4-Digit
Diagnostic Code criteria. The ARBD category has limited utility in
the diagnosis, but we do recognize that alcohol is teratogenic and
may be responsible for birth defects if exposure occurs during
critical periods of development. However, in the absence of other
features of FAS or brain deficits, it is difficult to attribute
causation.
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Future research related
to diagnostic guidelines |
The lack
or unavailability of evidence and data in key areas limits the
effectiveness of the diagnostic process, in general. Such key areas
include the development of Canadian growth and anthropometric norms
for all ages and ethno-cultural groups. There is also a need for the
development and validation of screening tools that are specific and
sensitive to prenatal alcohol exposure. These tools should be
adaptable for use in various contexts, they should be culturally
appropriate and they should lead to accurate referrals for diagnosis
and assessment.
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Emerging issues
|
Biomarkers
Often, women will not accurately recall the amount or frequency of
alcohol consumption during pregnancy. Some women may also
underestimate consumption level or deny that they drank alcohol
during pregnancy. Medical records are known to be incomplete
with respect to maternal alcohol history. Currently, there are
no reliable means to confirm maternal drinking using biochemical
markers in pregnancy. High levels of whole blood-associated
acetaldehyde, carbohydrate-deficient transferrin, gamma-glutamyl
transpeptidase and mean red blood cell volume may be useful
markers in pregnant women.50
Studies are underway to determine the utility of fatty acid ethyl
esters in meconium as markers for prenatal exposure to
alcohol.51,52,53
This marker will only be useful if it can be established that fatty
acid ethyl ester levels in meconium are predictive of developmental
outcome. Meconium testing could alert caregivers to infants who might
be at risk for alcohol effects and lead to appropriate monitoring,
intervention and prevention. Ethical issues regarding informed
consent surround the use of biological markers in the baby that may
indicate maternal drinking.
Recent innovations have led to the development of laser surface
scanning, a non-invasive method for acquiring 3-dimensional
images.33,34
This technique is promising in the analysis of facial features
associated with prenatal alcohol exposure, but, at present, is a
research tool only.
Remote and rural
areas
The availability of diagnostic services is limited in rural and
remote areas. A community may not have access to a diagnostic team or
resources and services. Until regionally based diagnostic teams are
established, the use of telemedicine for distant diagnosis,
consultation and training may be helpful.31
Recent advances using digital imaging and computer-assisted analysis
for the diagnosis of characteristic features of FAS have shown
promise for analysis of facial features associated with prenatal
alcohol exposure.32,33,44
Adult
diagnosis
Diagnosis of adults creates special challenges in all aspects of
the diagnosis. Physical features may change over time, there may be
catch-up growth, and cumulative environmental influences may distort
the evaluation of brain function. The adult's history may include
additional traumatic head injury, alcohol and drug abuse, and mental
health problems. Although tests for the various domains are readily
available, clinicians working with the adult FASD population find
that the tests are often not sensitive to real-life issues. In
addition to the data required for the diagnosis, an assessment must
include additional components such as functional literacy and
numeracy, employability and quality of life, which fall within the
domain of adaptive skills. The clinician should not rely solely on
the self-report of the individual who is alcohol-affected; the
history and abilities of the individual must be verified by a
reliable source.
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Conclusion
|
The
assessment for prenatal alcohol exposure is a diagnosis for the
affected person, the birth mother and possibly affected siblings.
Rather than labeling, a diagnosis provides a blueprint for early
intervention. Treatment planning and implementation, specifically
targeted toward the unique needs of the individual and the family,
form a large part of the diagnosis.
These guidelines and recommendations have been developed in
parallel and in consultation with a United States committee
charged with the same task.54 The
challenges for prevention and diagnosis of FASD and intervention to
assist those affected by this disorder are evolving and dynamic.
Research is ongoing to determine whether tools, such as novel brain
imaging techniques, biomarkers and DNA micro-array techniques, might
enhance accurate and reliable alcohol-related diagnoses and
treatment.
We hope that these guidelines and recommendations will be used to
facilitate training of health professionals, improve access to
diagnostic services and facilitate referral for intervention or
treatment for all people and families living with this disability.
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Appendix 1
|
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Appendix 2
|
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Appendix 3
|
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
Footnotes
|
*Astley SJ. Diagnostic Guide for Fetal Alcohol
Spectrum Disorders: The 4-Digit Diagnostic Code (3rd edition).
Seattle: University of Washington Publication Services; 2004.
 S1 -- Canadian Medical Association Journal_files/back.gif)
This article has been peer reviewed.
Contributors: All authors contributed equally to this manuscript.
All authors contributed substantially to conception and design,
or acquisition of data, or analysis and interpretation of data
and drafted the article or revised it critically for important
intellectual content. They gave their final approval of the
version submitted to be published.
Acknowledgements: This work is supported by the FASD Teams of
the Public Health Agency of Canada and the First Nations and
Inuit Health Branch, Health Canada. The authors would like to
thank the many clinicians and individuals who helped develop,
review and provide feedback on these guidelines, especially the
Public Health Agency of Canada's National Advisory Committee on FASD;
Drs. Fred Boland, Susan Astley and Sterling Clarren; and the Centers
for Disease Control and Prevention's scientific working group on
diagnosis. This work was supported by the Public Health Agency of
Canada and the First Nations and Inuit Health Branch, Health
Canada.
Competing interests: None declared.
 S1 -- Canadian Medical Association Journal_files/rarrow.gif) |
References
|
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in
early infancy. Lancet 1973;2(7836):999-1001.[Medline]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum
disorder. JAMA 2003;290(22):2996-9.[Free Full Text]
- Streissguth AP. Fetal alcohol syndrome: a guide for families
and communities. Baltimore: Paul H. Brookes; 1997.
- Stratton K, Howe C, Battaglia FC. Fetal alcohol syndrome:
diagnosis, epidemiology, prevention, and treatment. Washington: Institute
of Medicine and National Academy Press; 1996. Available: www.nap.edu/books/0309052920/html/index.html
(accessed 2004 Oct
29).
- Streissguth AP. Maternal drinking and the outcome of pregnancy:
implications for child mental health. Am J Orthopsychiatry
1977;47(3):422-31.[Medline]
- Joint statement: prevention of fetal alcohol syndrome (FAS) fetal
alcohol effects (FAE) in Canada. Ottawa: Health Canada; 1996.
- Roberts G., Nanson J. Best practices fetal alcohol
syndrome/fetal alcohol effects and the effects of other substance use during
pregnancy. Ottawa: Health Canada; 2003.
- Chavez GF, Cordero JF, Becerra JE. Leading major congenital
malformations among minority groups in the United States, 1981-1986. MMWR
Morb Mortal Wkly Rep 1988;37(3):17-24.[Medline]
- Sokol RJ, Clarren SK. Guidelines for use of terminology describing
the impact of prenatal alcohol on the offspring. Alcohol Clin Exp Res
1989;13(4):597-8.[Medline]
- Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Prenatal
alcohol exposure, birthweight, and measures of child size from birth to age 14
years. Am J Public Health 1994;84(9):1421-8.[Abstract]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK,
Dehaene P, et al. Incidence of fetal alcohol syndrome and prevalence of
alcohol-related neurodevelopmental disorder. Teratology
1997;56(5):317-26.[CrossRef][Medline]
- Robinson GC, Conry JL, Conry RF. Clinical profile and prevalence
of fetal alcohol syndrome in an isolated community in British Columbia.
CMAJ 1987; 137 (3):203-7.[Abstract]
- Williams RJ, Odaibo FS, McGee JM. Incidence of fetal alcohol
syndrome in northeastern Manitoba. Can J Public Health
1999;90(3):192-4.[Medline]
- Square D. Fetal alcohol syndrome epidemic on Manitoba reserve.
CMAJ 1997; 157(1):59-60.[Abstract/Free Full Text]
- Asante KO, Nelms-Maztke J. Report on the survey of children
with chronic handicaps and fetal alcohol syndrome in the Yukon and Northwest
British Columbia. Whitehorse: Council for Yukon Indians; 1985.
- Habbick BF, Nanson JL, Snyder RE, Casey RE, Schulman AL. Foetal
alcohol syndrome in Saskatchewan: unchanged incidence in a 20-year period.
Can J Pub Health 1996;87(3):204-7.[Medline]
- Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J,
Templin T, et al. Prenatal alcohol exposure and childhood behavior at age 6 to
7 years: I. dose-response effect. Pediatrics 2001;108(2):E34.
- Bingol N, Schuster C, Fuchs M, Iosub S, Turner G, Stone RK, et
al. The influence of socioeconomic factors on the occurrence of fetal alcohol
syndrome. Adv Alcohol Subst Abuse 1987;6(4):105-18.[Medline]
- Astley SJ, Bailey D, Talbot C, Clarren SK. Fetal alcohol syndrome
(FAS) primary prevention through FAS diagnosis: I. Identification of high-risk
birth mothers through the diagnosis of their children. Alcohol Alcohol
2000;35 (5): 499-508.[Abstract/Free Full Text]
- Jacobson JL, Jacobson SW. Prenatal alcohol exposure and
neurobehavioral development: where is the threshold? Alcohol Health Res
World 1994;18:30-6.
- Jacobson JL, Jacobson SW. Drinking moderately and pregnancy.
Effects on child development. Alcohol Res Health 1999;23(1):25-30.[Medline]
- Streissguth A, Barr H, Kogan J, Bookstein F. Primary and
secondary disabilities in fetal alcohol syndrome. In: Streissguth AP, Kanter
J, editors. The challenge of fetal alcohol syndrome: overcoming secondary
disabilities. Seattle: University of Washington Press; 1997. pp 25-39.
- Astley SJ, Bailey D, Talbot T, Clarren SK. Fetal alcohol syndrome
(FAS) primary prevention through FAS diagnosis: II. A comprehensive profile of
80 birth mothers of children with FAS. Alcohol Alcohol
2000;35(5):509-19.[Abstract/Free Full Text]
- Chudley AE, Longstaffe SE. Fetal alcohol syndrome and fetal
alcohol spectrum disorder. In: Cassidy S, Allanson J, editors. Management
of genetic syndromes. 2nd ed. New York: John Wiley and Sons; 2004.
- Astley SJ, Clarren SK. Diagnostic guide for fetal alcohol
syndrome and related conditions: the 4-Digit Diagnostic Code. 2nd ed.
Seattle: University of Washington Publication Services; 1999.
- Clarke ME, Tough SC. A national survey regarding knowledge and
attitudes of health professionals about fetal alcohol syndrome. Ottawa: Health
Canada; 2003.
- Lemoine P, Harousseau H, Borteyru JP, Menuet JC. Les enfants de
parents alcooliques - anomalies observées: à propos de 127 cas. Ouest
Med 1968; 21: 476-82.
- Jones KL, Smith DW, Ulleland CN, Streissguth AP. Pattern of
malformation in offspring of chronic alcoholic mothers. Lancet
1973;1(7815):1267-71.[Medline]
- Clarren SK, Smith DW. The fetal alcohol syndrome. Lamp
1978;35(10):4-7.[Medline]
- Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal
alcohol-exposed individuals: introducing the 4-Digit Diagnostic Code.
Alcohol Alcohol 2000;35 (4): 400-10.[Abstract/Free Full Text]
- Benoit T, Bowes MD, Bowman N, Cantin D, Chudley A, Crolly D, et
al. Telemedicine diagnosis for fetal alcohol syndrome - the Manitoba
experience. Pediatr Child Health 2002;7:147-51.
- Astley SJ, Stachowiak J, Clarren SK, Clausen C. Application of
the fetal alcohol syndrome facial photographic screening tool in a foster care
population. J Pediatr 2002;141(5):712-7.[CrossRef][Medline]
- Astley SJ, Clarren SK. Measuring the facial phenotype of
individuals with prenatal alcohol exposure: correlations with brain
dysfunction. Alcohol Alcohol 2001; 36 (2): 147-59.[Abstract/Free Full Text]
- Hennessy RJ, Kinsella A, Waddington JL. 3D laser surface scanning
and geometric morphometric analysis of craniofacial shape as an index of
cerebro-craniofacial morphogenesis: initial application to sexual dimorphism.
Biol Psychiatry 2002;51(6):507-14.[CrossRef][Medline]
- Da Silveira AC, Daw JL Jr, Kusnoto B, Evans C, Cohen M.
Craniofacial applications of three-dimensional laser surface scanning. J
Craniofac Surg 2003; 14 (4);449-56.
- US Department of Health and Human Services. National Institute on
Alcohol Abuse and Alcoholism. 10th special report to the US Congress on
alcohol and health: highlights from current research. Washington: The
Institute; 2000.
- Bradley KA, Boyd-Wickizer J, Powell SH, Burman ML. Alcohol
screening questionnaires in women: a critical review. JAMA
1998;280(2):166-71.[Abstract/Free Full Text]
- Russell M, Martier SS, Sokol RJ, Mudar P, Bottoms S, Jacobson S,
et al. Screening for pregnancy risk-drinking. Alcohol Clin Exp Res
1994;18(5):1156-61.[Medline]
- Astley SJ, Clarren SK. A case definition and photographic
screening tool for the facial phenotype of fetal alcohol syndrome. J
Pediatr 1996;129(1):33-41.[Medline]
- Jones KL, Smith DW. The fetal alcohol syndrome. Teratology
1975;12(1):1-10.[Medline]
- Thomas IT, Gaitantzis YA, Frias JL. Palpebral fissure length from
29 weeks gestation to 14 years. J Pediatr 1987;111:267-8.[Medline]
- Hall JG, Froster-Iskenius UG, Allanson JE, editors. Handbook
of normal physical measurements. Oxford: Oxford University Press; 1989.
pp. 149-50.
- Farkas LG. Anthropometry of the head and face. 2nd ed. New
York: Raven Press; 1994.
- Moore ES, Ward RE, Jamison PL, Morris CA, Bader PI, Hall BD. New
perspectives on the face in fetal alcohol syndrome: what anthropometry tells
us. Am J Med Genet 2002;109(4):249-60.[CrossRef][Medline]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy
prenatal alcohol exposure with or without physical features of fetal alcohol
syndrome leads to IQ deficits. J Pediatr 1997;131(5):718-21.[Medline]
- National population health survey, 1994-1995. Ottawa: Statistics
Canada; 1995. Available: stcwww.statcan.ca/english/sdds/5004.htm
(accessed 2004 Oct 29).
- National longitudinal survey of children and youth, 1994-1995.
Ottawa: Statistics Canada; 1995.
- National longitudinal survey of children and youth, 1998-1999.
Ottawa: Statistics Canada; 1999.
- Fall 2002 survey of First Nations people living on reserve.
Ottawa: EKOS Research; 2002.
- Stoler JM, Huntington KS, Petersen CM, Daniel P, Aboagye KK,
Lieberman E, et al. The prenatal detection of significant alcohol exposure
with maternal blood markers. J Pediatr 1998; 133(3):346-52.[Medline]
- Moore C, Jones J, Lewis D, Buchi K. Prevalence of fatty acid
ethyl esters in meconium specimens. Clin Chem 2003;49(1):133-6.[Abstract/Free Full Text]
- Bearer CF. Meconium as a biological marker of prenatal exposure.
Ambul Pediatr 2003;3(1):40-3.[CrossRef][Medline]
- Chan D, Bar-Oz B, Pellerin B, Paciorek C, Klein J, Kapur B, et
al. Population baseline of meconium fatty acid ethyl esters among infants of
non-drinking women in Jerusalem and Toronto. Ther Drug Monit
2003;25(3):271-8.[CrossRef][Medline]
- National Center on Birth Defects and Developmental Disabilities
Centers for Disease Control and Prevention Department of Health and Human
Services. Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis:
National Task Force on Fetal Alcohol Syndrome and Fetal Alcohol Effect; 2004
http://www.cdc.gov/ncbddd/fas/documents/FAS_guidelines_accessible.pdf
eLetters:
Read all eLetters
- On behalf of the Unheard Majority
- Barry stanley
- eCMAJ, 2 Mar 2005 [Full
text]